HHV-6 is a DNA virus belonging to the Herpesviridae family. Most people acquire Human Herpesvirus-6 (HHV-6) early in life where it usually remains in a latent state for the lifetime of the host. However, viral reactivation can occur at any time and cause serious complications in cases of transplantation and immune suppression.
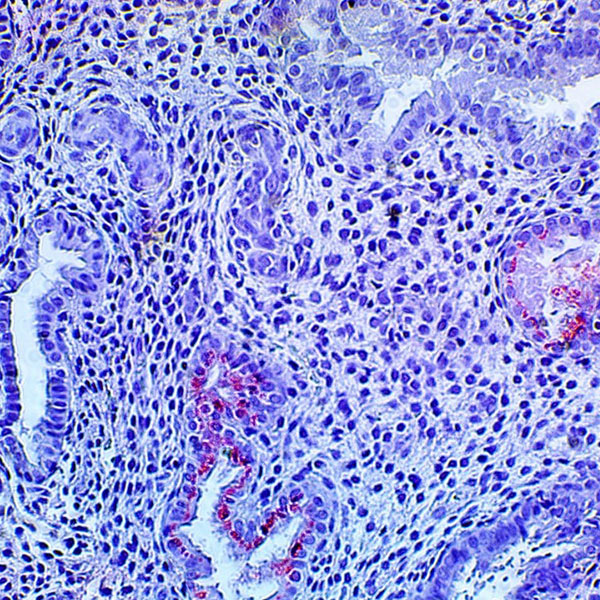
HHV-6 Immunohistochemistry
Immunohistochemical staining of tissues (IHC) is useful when analyzing tissue samples for active herpesvirus infections. The detection of HHV-6 late viral protein expression in tissues is definitive proof of active viral infection. Read more information on how IHC can be utilized in Transplant and Infertility:

Chromosomally Integrated HHV-6 (ciHHV-6)
Chromosomal Integration of HHV-6 (ciHHV-6) occurs in 0.5-2% of the population. The presence of the viral genome integrated into all body cells can confound diagnostic interpretation of HHV-6 DNA present in the blood, leading to a misdiagnosis of HHV-6 reactivation and unnecessary anti-viral treatment. Read more information on ci-HHV-6: