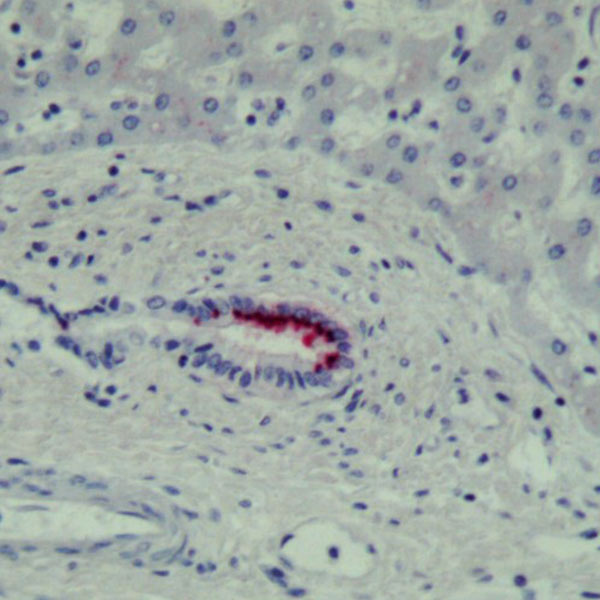
IHC/Transplant
Reactivation of HHV-6 after transplant is a common risk. The HHV-6 immunohistochemical staining (IHC) testing method on formalin-fixed paraffin embedded (FFPE) tissue is used to detect the presence of active HHV-6 infection in tissue.
Coppe Laboratories is the only reference laboratory in the U.S. providing HHV-6 IHC testing. We are a trusted provider of transplant centers and children’s hospitals throughout the nation. While posted turnaround time is 7 business days, STAT services can be arranged, as Coppe Laboratories recognizes the urgency in some cases. All HHV-6 IHC cases are reviewed by our board-certified pathologist to assess the pathological evidence of viral infection.
Published Research
Through our research, we were first to describe HHV-6 reactivation in transplant. A complete list of Coppe’s HHV-6 contributions and publications can be found here.
Related Reading
Immunohistochemical diagnosis of human infectious diseases: a review
Oumarou Hama H et al.
Immunohistochemistry (IHC) using monoclonal and polyclonal antibodies is a useful diagnostic method for detecting pathogen antigens in fixed tissues, complementing the direct diagnosis of infectious diseases by PCR and culture on fresh tissues.
2022, Diagn Pathol 17, 17
HHV-6 in liver transplantation: A literature review
Phan T L, Lautenschlager I, Razonable R R, Munoz F M
Some HHV-6 infections are localized in tissues without detectable DNA levels in blood ; Recipients with ciHHV-6 may have increased risk of infections and/or graft rejection
2017, Wiley Online Library
Association of Inherited Chromosomally Integrated Human Herpesvirus 6 with Neurologic Symptoms and Management after Allogeneic Hematopoietic Cell Transplantation
Heldman et al.
Testing for ciHHV-6 may improve patient management
2021, Science Direct
Human Herpesvirus 6 Infection in Pediatric Liver Transplantation: Single-Center Study of Incidence, Outcomes, and Management
Mysore et al.
HHV-6 serological status assessment pre-transplant may assist in risk stratification and post-transplant management
2021, JPIDS